Iso 9001 Corrective Action Examples
This article relies too much on to. Please improve this article by adding. (March 2012) () () The ISO 9000 family of standards is designed to help organizations ensure that they meet the needs of customers and other stakeholders while meeting statutory and regulatory requirements related to a product or program. 9000 deals with the fundamentals of quality management systems, including the seven quality management principles upon which the family of standards is based. ISO 9001 deals with the requirements that organizations wishing to meet the standard must fulfill.
Third-party certification bodies provide independent confirmation that organizations meet the requirements of ISO 9001. Over one million organizations worldwide are independently certified, making ISO 9001 one of the most widely used management tools in the world today. However, the ISO certification process has been criticized as being wasteful and not being useful for all organizations. Contents • • • • • • • • • • • • • • • • • • • • • Background [ ] ISO 9000 was first published in 1987 by ISO (). It was based on the BS 5750 series of standards from that were proposed to ISO in 1979. However, its history can be traced back some twenty years before that, to the publication of government procurement standards, such as the MIL-Q-9858 standard in 1959, and the U.K's 'Def Stan 05-21 and 05-24. Large organizations which supplied government procurement agencies often had to comply with a variety of quality assurance requirements for each contract awarded which led the defence industry to adopt mutual recognition of NATO AQAP, MIL-Q and Def Stan standards.
Eventually, ISO 9000 was adopted as a suitable option, instead of forcing contractors to adopt multiple - and often similar - requirements. Reasons for use [ ] The global adoption of ISO 9001 may be attributable to a number of factors. In the early days, the ISO 9001 (9002 and 9003) requirements were intended to be used by procuring organizations, as the basis of contractual arrangements with their suppliers. This helped reduce the need for 'supplier development' by establishing basic requirements for a supplier to assure product quality.
The ISO 9001 requirements could be tailored to meet specific contractual situations, depending the complexity of product, business type (design responsibility, manufacture only, distribution, servicing etc) and risk to the procurer. If a chosen supplier was weak on the controls of their measurement equipment (calibration), and hence QC/inspection results, that specific requirement would be invoked in the contract. The adoption of a single Quality Assurance requirement also lead to cost savings throughout the supply chain by reducing the administrative burden of maintaining multiple sets of quality manuals and procedures. A few years later, the U.K. Government took steps to improve national competitiveness following publication of cmd 8621, and Third Party Certification of Quality Management Systems was born, under the auspices of the National Accreditation Council of Certification Bodies (NACCB) which has become the United Kingdom Accreditation Service (UKAS).
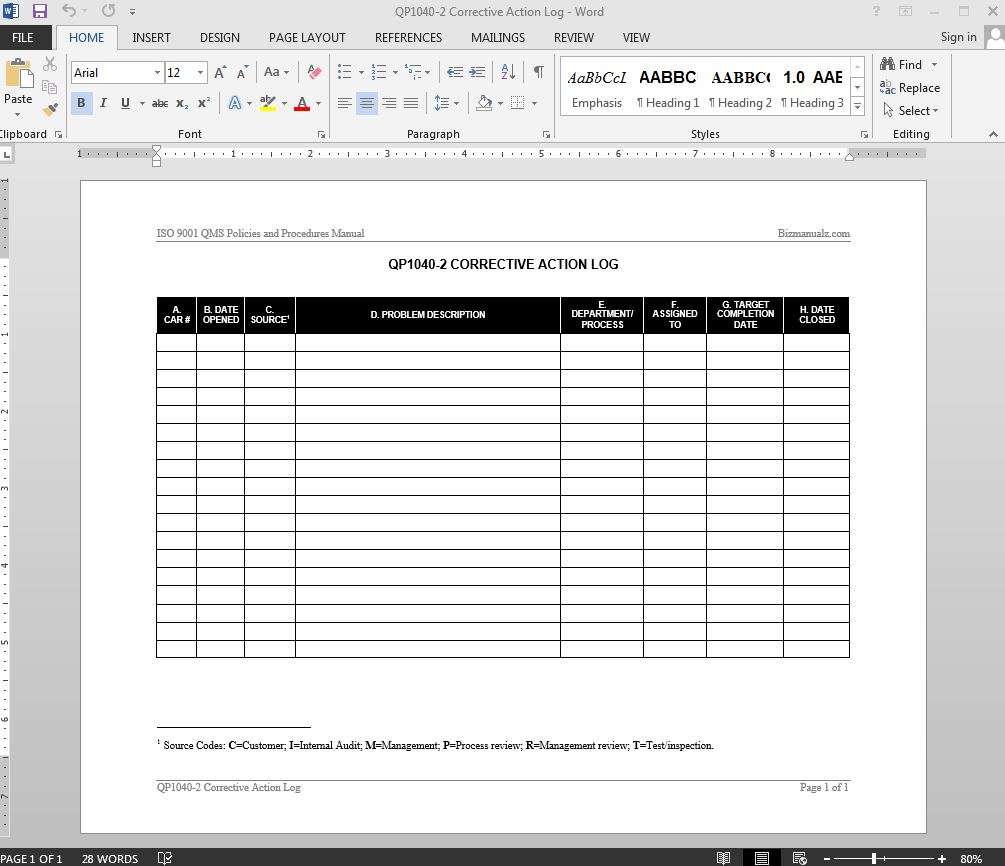
Corrective and preventive action (CAPA, also called Corrective Action / Preventive Action, or simply Corrective Action) are improvements to an organization's.
In addition to several stakeholders' benefits, a number of studies have identified significant financial benefits for organizations certified to ISO 9001, with a 2011 survey from the British Assessment Bureau showing 44% of their certified clients had won new business. Corbett et al. Showed that certified organizations achieved superior compared to otherwise similar organizations without certification. Found similarly superior performance and demonstrated that this was statistically significant and not a function of organization size.
Naveha and Marcus claimed that implementing ISO 9001 led to superior operational performance in the. Sharma identified similar improvements in operating performance and linked this to superior financial performance. Chow-Chua et al. Showed better overall financial performance was achieved for companies in.
Rajan and Tamimi (2003) showed that ISO 9001 certification resulted in superior stock market performance and suggested that shareholders were richly rewarded for the investment in an ISO 9001 system. While the connection between superior financial performance and ISO 9001 may be seen from the examples cited, there remains no proof of direct causation, though, such as those of Corbett et al. (2005) may suggest it. Other writers, such as Heras et al. (2002), have suggested that while there is some evidence of this, the improvement is partly driven by the fact that there is a tendency for better performing companies to seek ISO 9001 certification. The mechanism for improving results has also been the subject of much research.
(2007) identified operational improvements (e.g., cycle time reduction, inventory reductions) as following from certification. [ ] Internal process improvements in organizations lead to externally observable improvements. The benefit of increased international trade and domestic market share, in addition to the internal benefits such as customer satisfaction, interdepartmental communications, work processes, and customer/supplier partnerships derived, far exceeds any and all initial investment. Global adoption [ ] The increase in ISO 9001 certification is shown in the tables below. Worldwide total of ISO 9001 certificates (end of each year) 2000 2001 2002 2003 2004 2005 2006 2007 409,421 510,616 561,747 567,985 660,132 773,867 896,929 951,486 2008 2009 2010 2011 2012 2013 2014 982,832 1,064,785 1,118,510 1,111,698 1,096,987 1,126,460 1,138,155 Top 10 countries for ISO 9001 certificates (2014) Rank Country No. Of certificates 1 China 342,800 2 Italy 168,960 3 Germany 55,363 4 Japan 45,785 5 India 41,016 6 United Kingdom 40,200 7 Spain 36,005 8 United States 33,008 9 France 29,122 10 Australia 19,731 Top 10 countries for ISO 9001 certificates (2010) Rank Country No.
Of certificates 1 China 297,037 2 Italy 138,892 3 Russian Federation 62,265 4 Spain 59,854 5 Japan 59,287 6 Germany 50,583 7 United Kingdom 44,849 8 India 33,250 9 United States 25,101 10 Korea, Republic of 24,778 Top 10 countries for ISO 9001 certificates (2009) Rank Country No. A fish wholesaler in, advertising its ISO 9001 certification. ISO 9001:2015 Quality management systems — Requirements is a document of approximately 30 pages which is available from the national standards organization in each country. Only ISO 9001 is directly audited against for third party assessment purposes. Contents of ISO 9001:2015 are as follows: • Section 1: Scope • Section 2: Normative references • Section 3: Terms and definitions • Section 4: Context of the organization • Section 5: Leadership • Section 6: Planning • Section 7: Support • Section 8: Operation • Section 9: Performance evaluation • Section 10: Improvement Essentially the layout of the standard is similar to the previous ISO 9001:2008 standard in that it follows the cycle in a process based approach, but is now further encouraging this to have risk based thinking. (section 0.3.3 of the introduction) The purpose of the quality objectives is to determine the conformity of the requirements (customers and organizations), facilitate effective deployment and improve the quality management system. Before the certification body can issue or renew a certificate, the auditor must be satisfied that the company being assessed has implemented the requirements of sections 4 to 10.
Sections 1 to 3 are not directly audited against, but because they provide context and definitions for the rest of the standard, not that of the organization, their contents must be taken into account. The standard no longer specifies that the organization shall issue and maintain documented procedures, however ISO 9001:2015 requires the organization to document any other procedures required for its effective operation. The standard also requires the organization to issue and communicate a documented, a quality management system scope, and quality objectives. The standard no longer requires compliant organizations to issue a formal Quality Manual. The standard does require retention of numerous records, as specified throughout the standard. New for the 2015 release is a requirement for an organization to assess risks and opportunities (section 6.1) and to determine internal and external issues relevant to its purpose and strategic direction (section 4.1).
The organization must demonstrate how the standard’s requirements are being met, while the external auditor’s role is to determine the quality management system's effectiveness. More detailed interpretation and implementation examples are often sought by organizations seeking more information in what can be a very technical area. Certification [ ] does not certify organizations itself. Numerous certification bodies exist, which audit organizations and, upon success, issue ISO 9001 compliance certificates. Although commonly referred to as 'ISO 9000' certification, the actual standard to which an organization's quality management system can be certified is ISO 9001:2015 (ISO 9001:2008 will expire by around September 2018). Many countries have formed bodies to authorize ('accredit') the certification bodies.
Both the accreditation bodies and the certification bodies charge fees for their services. The various accreditation bodies have mutual agreements with each other to ensure that certificates issued by one of the (CB) are accepted worldwide. Certification bodies themselves operate under another quality standard, ISO/IEC 17021, while accreditation bodies operate under ISO/IEC 17011. An organization applying for ISO 9001 certification is audited based on an extensive sample of its sites, functions, products, services and processes. The auditor presents a list of problems (defined as 'nonconformities', 'observations', or 'opportunities for improvement') to management. If there are no major nonconformities, the certification body will issue a certificate.
Where major nonconformities are identified, the organization will present an improvement plan to the certification body (e.g., corrective action reports showing how the problems will be resolved); once the certification body is satisfied that the organization has carried out sufficient corrective action, it will issue a certificate. The certificate is limited by a certain scope (e.g., production of golf balls) and will display the addresses to which the certificate refers.
An ISO 9001 certificate is not a once-and-for-all award, but must be renewed at regular intervals recommended by the certification body, usually once every three years. There are no grades of competence within ISO 9001: either a company is certified (meaning that it is committed to the method and model of quality management described in the standard) or it is not.
In this respect, ISO 9001 certification contrasts with measurement-based quality systems. Evolution of ISO 9000 standards [ ] The ISO 9000 standard is continually being revised by standing technical committees and advisory groups, who receive feedback from those professionals who are implementing the standard.
1987 version [ ] ISO 9000:1987 had the same structure as the UK Standard BS 5750, with three 'models' for quality management systems, the selection of which was based on the scope of activities of the organization: • ISO 9001:1987 Model for quality assurance in design, development, production, installation, and servicing was for companies and organizations whose activities included the creation of new products. • ISO 9002:1987 Model for quality assurance in production, installation, and servicing had basically the same material as ISO 9001 but without covering the creation of new products. • ISO 9003:1987 Model for quality assurance in final inspection and test covered only the final inspection of finished product, with no concern for how the product was produced. ISO 9000:1987 was also influenced by existing U.S. And other ('MIL SPECS'), and so was well-suited to manufacturing.
The emphasis tended to be placed on conformance with procedures rather than the overall process of management, which was likely the actual intent. [ ] 1994 version [ ] ISO 9000:1994 emphasized via preventive actions, instead of just checking final product, and continued to require evidence of compliance with documented procedures. As with the first edition, the down-side was that companies tended to implement its requirements by creating shelf-loads of procedure manuals, and becoming burdened with an ISO bureaucracy. In some companies, adapting and improving processes could actually be impeded by the quality system.
[ ] 2000 version [ ] ISO 9001:2000 replaced all three former standards of 1994 issue, ISO 9001, ISO 9002 and ISO 9003. Design and development procedures were required only if a company does in fact engage in the creation of new products. The 2000 version sought to make a radical change in thinking by actually placing front and centre the concept of (the monitoring and optimisation of a company's tasks and activities, instead of just inspection of the final product).
The 2000 version also demanded involvement by upper executives in order to integrate quality into the business system and avoid delegation of quality functions to junior administrators. Another goal was to improve effectiveness via process performance metrics: numerical measurement of the effectiveness of tasks and activities. Expectations of continual process improvement and tracking customer satisfaction were made explicit. ISO 9000 Requirements include: • Approve documents before distribution; • Provide correct version of documents at points of use; • Use your records to prove that requirements have been met; and • Develop a procedure to control your records. 2008 version [ ] ISO 9001:2008 in essence re-narrates ISO 9001:2000. The 2008 version only introduced clarifications to the existing requirements of ISO 9001:2000 and some changes intended to improve consistency with. There were no new requirements.
For example, in ISO 9001:2008, a quality management system being upgraded just needs to be checked to see if it is following the clarifications introduced in the amended version. ISO 9001 is supplemented directly by two other standards of the family: • ISO 9000:2005 'Quality management systems. Fundamentals and vocabulary' • ISO 9004:2009 'Managing for the sustained success of an organization.
A quality management approach' Other standards, like and the ISO 10000 series, may also be used for specific parts of the quality system. ISO9001-2015 version [ ] In 2012, ISO TC 176 - responsible for ISO 9001 development - celebrated 25 years of implementing ISO 9001, and concluded that it is necessary to create a new QMS model for the next 25 years. This is why they commenced the official work on creating a revision of ISO 9001, starting with the new QM principles. This moment was considered by important specialists in the field as 'beginning of a new era in the development of quality management systems.'
As a result of the intensive work from this technical committee, the revised standard ISO 9001:2015 was published by ISO on 23 September 2015. The scope of the standard has not changed; however, the structure and core terms were modified to allow the standard to integrate more easily with other international management systems standards. The 2015 version is also less prescriptive than its predecessors and focuses on performance.
This was achieved by combining the process approach with risk-based thinking, and employing the Plan-Do-Check-Act cycle at all levels in the organization. Some of the key changes include: • High Level Structure of 10 clauses is implemented. Now all new standard released by ISO will have this High level structure. This section possibly contains.
Please by the claims made and adding. Statements consisting only of original research should be removed. (May 2013) () The debate on the effectiveness of ISO 9000 commonly centers on the following questions: • Are the quality principles in ISO 9001 of value? • Does it help to implement an ISO 9001-compliant quality management system?
• Does it help to obtain ISO 9001 certification? Effectiveness of the ISO system being implemented depends on a number of factors, the most significant of which are: • Commitment of senior management to monitor, control, and improve quality. Organizations that implement an ISO system without this desire and commitment often take the cheapest road to get a certificate on the wall and ignore problem areas uncovered in the audits. • How well the ISO system integrates into current business practices. Many organizations that implement ISO try to make their system fit into a cookie-cutter quality manual instead of creating a manual that documents existing practices and only adds new processes to meet the ISO standard when necessary. • How well the ISO system focuses on improving the customer experience.
The broadest definition of quality is 'Whatever the customer perceives good quality to be.' This means that a company doesn't necessarily have to make a product that never fails; some customers will have a higher tolerance for product failures if they always receive shipments on-time or have a positive experience in some other dimension of customer service. An ISO system should take into account all areas of the customer experience and the industry expectations, and seek to improve them on a continual basis. This means taking into account all processes that deal with the three stakeholders (customers, suppliers, and organization); only then will a company be able to sustain improvements in the customer's experience.
• How well the auditor finds and communicates areas of improvement. While ISO auditors may not provide consulting to the clients they audit, there is the potential for auditors to point out areas of improvement. Many auditors simply rely on submitting reports that indicate compliance or non-compliance with the appropriate section of the standard; however, to most executives, this is like speaking a foreign language. Auditors that can clearly identify and communicate areas of improvement in language and terms executive management understands facilitate action on improvement initiatives by the companies they audit. When management doesn't understand why they were non-compliant and the business implications associated with non-compliance, they simply ignore the reports and focus on what they do understand.
Advantages [ ] Proper quality management can improve business, often having a positive effect on investment, market share, sales growth, sales margins, competitive advantage, and avoidance of litigation. The quality principles in ISO 9000:2000 are also sound, according to Wade and Barnes, who says that 'ISO 9000 guidelines provide a comprehensive model for quality management systems that can make any company competitive'. Sroufe and Curkovic, (2008) found benefits ranging from registration required to remain part of a supply base, better documentation, to cost benefits, and improved involvement and communication with management.
According to ISO the 2015 version of the standard brings the following benefits: • By assessing their context, organizations can define who is affected by their work and what they expect. This enables clearly stated business objectives and the identification of new business opportunities. • Organizations can identify and address the risks associated with their organization. • By putting customers first organizations can make sure they consistently meet customer needs and enhance customer satisfaction. This can lead to more repeat custom, new clients and increased business for the organization. • Organizations work in a more efficient way as all their processes are aligned and understood by everyone. This increases productivity and efficiency, bringing internal costs down.
• Organizations will meet necessary statutory and regulatory requirements. • Organizations can expand into new markets, as some sectors and clients require ISO 9001 before doing business. Criticisms of ISO 9000 [ ] A common criticism of ISO 9000 and 9001 is the amount of money, time, and paperwork required for registration. Dalgleish cites the 'inordinate and often unnecessary paperwork burden' of ISO, and says that 'quality managers feel that ISO's overhead and paperwork are excessive and extremely inefficient'. According to Barnes, 'Opponents claim that it is only for documentation. Proponents believe that if a company has documented its quality systems, then most of the paperwork has already been completed'. Wilson suggests that ISO standards 'elevate inspection of the correct procedures over broader aspects of quality', and therefore, 'the workplace becomes oppressive and quality is not improved'.
One study showing reasons for not adopting this standard include the risks and uncertainty of not knowing if there are direct relationships to improved quality, and what kind and how many resources will be needed. Additional risks include how much certification will cost, increased bureaucratic processes and risk of poor company image if the certification process fails. Bomb The Suburbs Pdf. According to, ISO 9001 promotes specification,, and procedures rather than and improvement. Wade argues that ISO 9000 is effective as a guideline, but that promoting it as a standard 'helps to mislead companies into thinking that certification means better quality. [undermining] the need for an organization to set its own quality standards'. In short, Wade argues that reliance on the specifications of ISO 9001 does not guarantee a successful quality system.
The standard is seen as especially prone to failure when a company is interested in certification before quality. Certifications are in fact often based on customer contractual requirements rather than a desire to actually improve quality. 'If you just want the certificate on the wall, chances are you will create a paper system that doesn't have much to do with the way you actually run your business', said ISO's Roger Frost. Certification by an independent auditor is often seen as the problem area, and according to Barnes, 'has become a vehicle to increase consulting services'.
Dalgleish argues that while 'quality has a positive effect on return on investment, market share, sales growth, better sales margins and competitive advantage,' 'taking a quality approach is unrelated to ISO 9000 registration.' In fact, ISO itself advises that ISO 9001 can be implemented without certification, simply for the quality benefits that can be achieved. Abrahamson argues that fashionable management discourse such as tends to follow a in the form of a, possibly indicating a.
Pickrell argues [ ] that ISO systems merely gauge whether the processes are being followed. It does not gauge how good the processes are or whether the correct parameters are being measured and controlled to ensure quality. Furthermore, when unique technical solutions are involved in the creation of a new part, ISO does not validate the robustness of the technical solution which is a key part of advanced quality planning. It is not unheard of for an ISO-certified plant to display poor quality performance due to poor process selection and/or poor technical solutions.
See also [ ] • —Containing published standards • —Quality management—Guidelines to quality management in projects • —Medical devices -- Quality management systems -- Requirements for regulatory purposes • —Environmental management standards • —Guidelines for quality management systems auditing and environmental management systems auditing • —Quality management system requirements for automotive-related products suppliers • —Information security management • —Road traffic safety management • —Energy Audit • —aerospace industry implementation of ISO 9000/1 • • • • • • References [ ].
Khg Team Sony Vegas Crack. Corrective and preventive action ( CAPA, also called Corrective Action / Preventive Action, or simply Corrective Action) are improvements to an organization's processes taken to eliminate causes of or other undesirable situations. It is usually a set of actions that laws or regulations require an organization to take in manufacturing, documentation, procedures, or systems to rectify and eliminate recurring nonperformance.
Nonperformance is identified after systematic evaluation and analysis of the root cause of the non-conformance. Non-conformance may be a market complaint or customer complaint or a failure of a machinery or a quality management system, or misinterpretation of written instructions to carry out a work. The corrective and preventive action is designed by a team that includes quality assurance personnel and personnel involved in the actual observation point of nonconformance.
It must be systematically implemented and observed for its ability to eliminate further recurrence of such non-conformation. In certain markets and industries, CAPA may be required as part of the quality management system, such as the Medical Devices and Pharmaceutical industries in the United States. In this case, failure to adhere to proper CAPA handling is considered a violation of US Federal regulations on good manufacturing practices. As a consequence, a medicine or medical device can be termed as adulterated or substandard if the company has failed to investigate, record and analyse the root-cause of a non-conformance, and failed to design and implement an effective CAPA.
CAPA is used to bring about improvements to an organization's processes, and is often undertaken to eliminate causes of or other undesirable situations. CAPA is a concept within (GMP), / (/) and numerous business standards. It focuses on the systematic investigation of the of identified problems or identified in an attempt to prevent their recurrence (for corrective action) or to prevent occurrence (for preventive action). Corrective actions are implemented in response to customer complaints, unacceptable levels of product non-conformance, issues identified during an, as well as adverse or unstable trends in product and process monitoring such as would be identified by (SPC). Preventive actions are implemented in response to the identification of potential sources of non-conformity. To ensure that corrective and preventive actions are effective, the systematic investigation of the root causes of failure is pivotal.
CAPA is part of the overall (QMS). The PDCA cycle Preventive action is any proactive methodology used to determine potential discrepancies before they occur and to ensure that they do not happen (thereby including, for example, preventive maintenance, management review or other common forms of risk avoidance). Corrective and preventive actions both include stages for investigation, action, review, and further action if required. It can be seen that both fit into the (plan-do-check-act) philosophy as determined by the Deming-Shewhart cycle. Investigations to root cause may conclude that no corrective or preventive actions are required, and additionally may suggest simple corrections to a problem with no identified systemic root cause. When multiple investigations end in no corrective action, a new problem statement with expanded scope may be generated, and a more thorough investigation to root cause performed. Implementation of corrective and preventive actions is the path towards improvement and effectiveness of Quality Management Systems.
Corrective actions is nothing but the action/actions based on the problem identification. The problem or a non-conformance can be identified internally through staff suggestions, management reviews, document reviews or internal audits. External leads to finding the root cause of the problem can include: Customer complaints/suggestions; customer rejections; non-conformities raised in customer/third-party audits; recommendations by auditors.
A root cause is the identification of the source of the problem where the person(s), system, process, or external factor is identified as the cause of the non conformity. The root cause analysis can be done via or other methods, e.g. Corrective action is the re-work/rectification activity of the non-conforming products as per ISO 9001:2008 (8.5.2). Preventive action includes the prediction of problems and attempts to avoid such occurrences (fail safe) through self-initiated actions and analysis related to the processes/products. This can be initiated with the help of an active participation by staff members/workers through improvement teams, improvement meetings, opportunities for improvement during internal audits, management review, customer feedback and deciding own goals quantized in terms of business growth, reducing rejections, utilizing the equipment effectively, etc. Medical devices and FDA compliance [ ] To comply with the United States 's code FDA 21 CFR 820.100 medical device companies need to establish a CAPA process within their QMS. This part of the system may be paper or digital, but it is something that is looked for during an FDA visit.
In 2015 there were over 450 issues found with the CAPA systems for medical device companies. To have an FDA-compliant QMS system required the ability to capture, review, approve, control, and retrieve closed-loop processes. Examples of corrective actions [ ] • Error Proofing • Visible or Audible Alarms • Process Redesign • Product Redesign • or enhancement/ modification of existing training programmes • Improvements to schedules • Improvements to material handling or storage In some cases, a combination of such actions may be necessary to fully correct the problem.
See also [ ] • • • • (GAMP) References [ ].